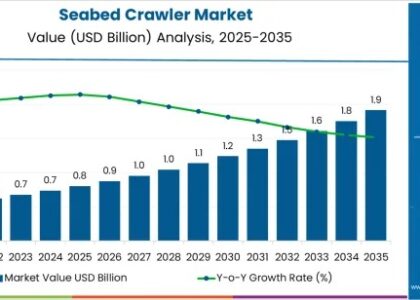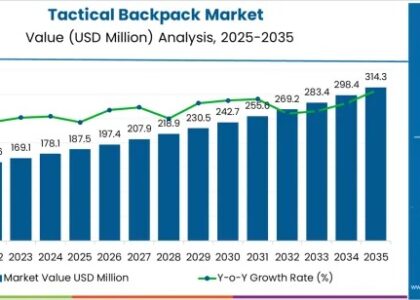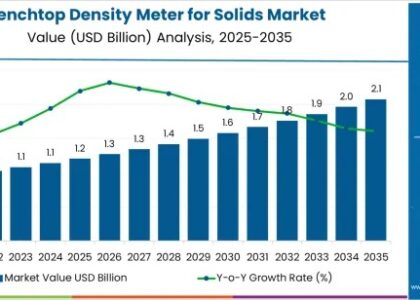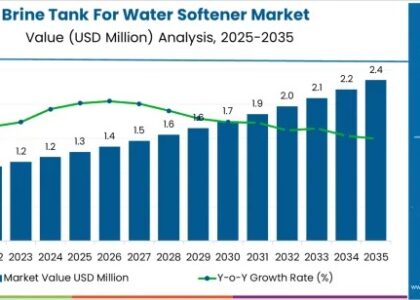
The global primary hyperoxaluria (PH) treatment market is projected to achieve a market value of USD 18 million in 2023, with expectations to grow significantly to USD 41.07 million by 2033. This growth reflects a healthy CAGR of 8.6% during the forecast period from 2023 to 2033, highlighting the increasing focus on effective treatments for this rare genetic disorder.
Primary hyperoxaluria is characterized by the liver’s inability to properly metabolize oxalate, resulting in elevated oxalate levels that can lead to the formation of kidney stones and progressive kidney damage. As awareness of this condition grows, so does the demand for innovative drugs and therapies designed to reduce oxalate production, manage symptoms, and prevent complications associated with the disorder.
In the historical period from 2018 to 2022, the primary hyperoxaluria treatment market registered a CAGR of 5.2%, reflecting a gradual increase in research and development efforts aimed at addressing this complex condition. Advances in genetic therapies and novel treatment modalities are expected to drive further growth in the coming years, offering hope for patients affected by PH.
Primary hyperoxaluria (PH) is a rare genetic disorder that affects the liver’s ability to metabolize oxalate, leading to the formation of kidney stones and progressive kidney damage. The primary hyperoxaluria treatment market includes drugs and therapies aimed at reducing the production of oxalate in the body, managing symptoms, and preventing complications.
Some of the primary hyperoxaluria treatments available in the market include Lumasiran (Oxlumo), an RNA interference (RNAi) therapeutic that works by blocking the production of the enzyme that produces oxalate in the liver. The drug was approved by the FDA in 2020 for the treatment of PH type 1 in children and adults; Oxalate-degrading enzymes that can break down oxalate in the gut, reducing the amount of oxalate that is absorbed into the bloodstream; Kidney stone management and Dialysis and kidney transplant.
The primary hyperoxaluria treatment market is relatively small due to the rarity of the disease, but with the approval of Lumasiran, there is a growing focus on developing new therapies for the condition. Some companies currently working on PH treatments include Dicerna Pharmaceuticals, Alnylam Pharmaceuticals, and Allena Pharmaceuticals.
What are the Challenges Faced by the Primary Hyperoxaluria Treatment Market?
Expensive Cost of Treatment to restrict Market Growth
One of the significant challenges faced by the PKU therapeutics market is the lack of awareness and underdiagnosis of the disorder. Primary hyperoxaluria is a rare disease, and as a result, there is limited awareness of the condition among healthcare providers and the general public. This can lead to delays in diagnosis and treatment, which can negatively impact patient outcomes.
Moreover, developing new treatments for rare diseases such as primary hyperoxaluria can be challenging, as the regulatory environment can be complex and uncertain. This can lead to delays in bringing new therapies to market. Overall, while there have been some recent advances in the primary hyperoxaluria treatment market, there are still several challenges that need to be addressed to improve patient outcomes and ensure the development of more effective and accessible treatments.
Market Competition
Key players in the market include companies such as Competition Deep Dive, Alnylum Pharma, OxThera, Dicerna Pharmaceuticals, Inc., Allena Pharmaceuticals, Biocodex, Tecoland Corporation, Zhejiang Tianxin Pharmaceutical Co., Takeda Pharmaceuticals, Wuxi Further Pharmaceutical Co Ltd, Genentech, along with healthcare providers and technology companies among other global players.
- In 2020, The United States Food and Drug Administration (FDA) gave the green light to Oxlumo (lumasiran) as the initial treatment for primary hyperoxaluria type 1 (PH1), a rare hereditary disorder. This groundbreaking approval is the culmination of the work of specialists and community members coordinated by the Oxalosis & Hyperoxaluria Foundation and the Kidney Health Initiative.
- The approval of Oxlumo was a result of input from patients, treating physicians, experts, and sponsors at a patient-focused drug development meeting and through other collaborative efforts. Oxlumo’s function is to reduce the production of oxalate in patients with PH1. The drug was evaluated in two separate trials in PH1 patients: a randomized, placebo-controlled trial in patients aged six years and older, and an open-label study in patients under six years old. The patients ranged in age from four months to 61 years at the start of treatment.
Key Companies Profiled
- Alnylum Pharma
- OxThera
- Dicerna Pharmaceuticals Inc.
- Allena Pharmaceuticals
- Biocodex
- Tecoland Corporation
- Zhejiang Tianxin Pharmaceutical Co.
- Takeda Pharmaceuticals
- Wuxi Further Pharmaceutical Co Ltd
- Genentech
Key Segments Profiled in the Primary Hyperoxaluria Treatment Industry Survey
Type:
- Type 1
- Type 2
- Type 3
Drug Class:
- Pyridoxine
- Potassium Citrate
- Thiazides
- Orthophosphates
Route of Administration:
- Oral
- Intravenous
Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Region:
- North America
- Latin America
- Europe
- East Asia
- South Asia
- Oceania
- Middle East & Africa
Author By:
Sabyasachi Ghosh (Associate Vice President at Future Market Insights, Inc.) holds over 12 years of experience in the Healthcare, Medical Devices, and Pharmaceutical industries. His curious and analytical nature helped him shape his career as a researcher.
Identifying key challenges faced by clients and devising robust, hypothesis-based solutions to empower them with strategic decision-making capabilities come naturally to him. His primary expertise lies in areas such as Market Entry and Expansion Strategy, Feasibility Studies, Competitive Intelligence, and Strategic Transformation.
Holding a degree in Microbiology, Sabyasachi has authored numerous publications and has been cited in journals, including The Journal of mHealth, ITN Online, and Spinal Surgery News.
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube