The global dystrophic epidermolysis bullosa (DEB) management market is set to experience substantial growth, with a projected market value of USD 452.37 Million in 2023, according to the latest report. The market is anticipated to expand at a compound annual growth rate (CAGR) of 5.7% from 2023 to 2033, driven by the increasing prevalence of skin diseases and a high incidence of dystrophic epidermolysis bullosa cases.
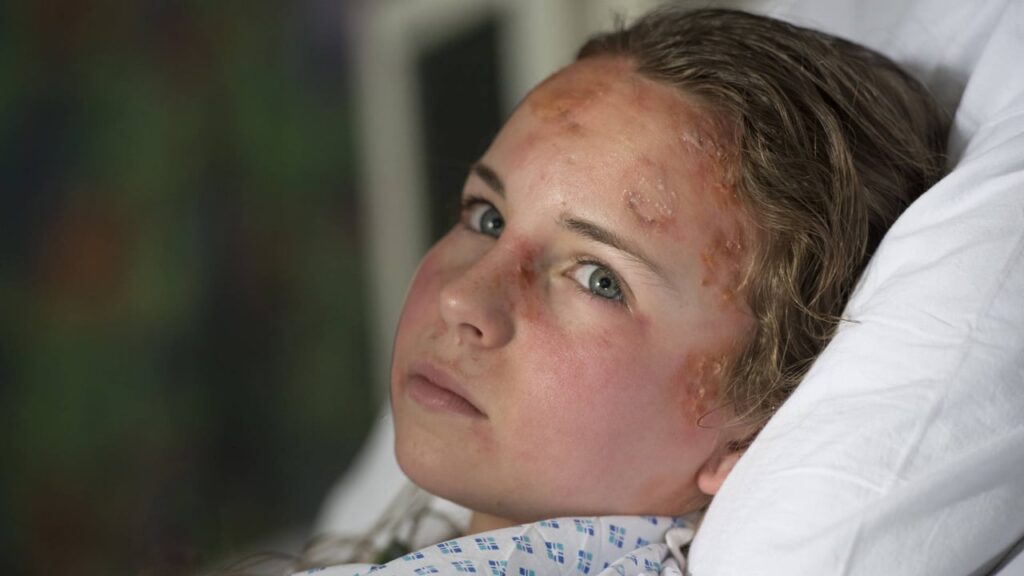
Dystrophic epidermolysis bullosa is a rare but severe genetic skin condition that causes fragile skin prone to blistering. The high incidence of this condition, combined with growing awareness and advancements in treatment options, are significant factors propelling the market’s growth.
Treatment for dystrophic epidermolysis bullosa involves a wound care regimen that integrates contemporary wound care techniques while eliminating factors that can irritate and inflame the skin. Dermatologists recommend adhering to strict personal hygiene habits and thoroughly moisturizing after treatments for dystrophic epidermolysis bullosa to promote healing of blisters without further damage or infection. Aqueous disinfectants are highly effective in treating DEB, and micro-adhesive silicone-based wound care is particularly useful for individuals with tough skin areas like elbows, shoulders, and trunks. Topical antibiotic ointments are expected to become the primary treatment for curing wounds and preventing the spread of infection in the future.
Various methods are being researched to treat EB, including the use of gene-corrected iPS cells from the patient’s own body, gene editing technologies, and polymer-mediated DNA delivery methods. Protein replacement therapies have been successful in treating several genetic diseases and could potentially be used to treat EB. A protein therapy approach involving intravenous and intradermal injections of recombinant collagen VII appears to be promising in treating DEB at the preclinical level, but further testing and development are necessary to determine its usefulness in treating patients.
Key Takeaways:
- By 2023, global dystrophic epidermolysis bullosa management sales are expected to reach US$ 452.37 Million.
- Antibiotics for dystrophic epidermolysis bullosa management are expected to grow 32% market share between 2023 and 2033.
- Hospital pharmacies for the dystrophic epidermolysis bullosa management market are projected to grow at a CAGR of 3.4% from 2023 to 2033.
- A market share of 23% is forecasted for the North American dystrophic epidermolysis bullosa management market.
- European markets are projected to grow at a CAGR of 2.6% during the forecast period.
“The development of stem cell therapy, approval of new topical and oral medicines, and development of diagnostic devices will support the growth of dystrophic epidermolysis bullosa management market.” comments a Future Market Insights analyst.
Competitive Landscape :
Diagnostic techniques are increasingly being developed and researched in parallel as they advance. Several companies will likely expand their product lines, acquire other firms, or merge in the near future as new techniques and treatment emerge.
- Abeona Therapeutics Inc. announced the completion of the final 6-month follow-up visit of its primary Phase 3 VIITAL trial of EB-101, the company’s targeted cell therapy investigation for dystrophic epidermolysis bullosa (RDEB). In order to lock the database within two to three weeks after the last patient’s final visit, Abeona has been verifying and preparing the data in real-time. As a result of the VIITAL study, Abeona intends to seek approval from the U.S. Food and Drug Administration (FDA) for EB-101 for the treatment of RDEB patients.
- Researchers from Relief Therapeutics will test APR-TD011 on the effects of bacterial colonization on epidermolysis bullosa (EB) wounds in a pilot study. A chemical with strong antimicrobial properties, hypochlorous acid, is the active ingredient in the treatment. Buying Nexodyn helps cleanse and moisturize cuts, burns, and abrasions as well as acute wounds. In a trial that has been initiated by the investigator (NCT05533866), 15 patients with junctional EB or dystrophic EB and wounds infected with Staphylococcus aureus and/or Pseudomonas aeruginosa bacteria will be enrolled soon.
Key players:
Castle Creek Biosciences, Amryth Pharma, Krystal Biotech, Abeona Therapeutics, BridgeBio, Phoenix Tissue Repair, Wings Therapeutics, InMed Pharmaceuticals, Inc., Regenerx Biopharmaceuticals Inc., Holostem Terapie Avanzate S.r.l
More insights available
In North America and Europe, where there is a greater prevalence of the condition and higher levels of healthcare spending, there are more treatment options available. These options include wound care management, pain management, and gene therapy. The U.S. Food and Drug Administration (FDA) has approved several products for Dystrophic Epidermolysis Bullosa treatment, including a topical gel that helps to manage blistering and wound healing.
A Old Full Report Analysis Click Here
Key Segments:
By Disease Type:
- Dominant Dystrophic Epidermolysis Bullosa (DDEB)
- Recessive Dystrophic Epidermolysis Bullosa (RDEB)
By Drug Class:
- Antibiotics
- Corticosteroids
- Opioid Analgesics
- Anticonvulsant
By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Author By:
Sabyasachi Ghosh (Associate Vice President at Future Market Insights, Inc.) holds over 12 years of experience in the Healthcare, Medical Devices, and Pharmaceutical industries. His curious and analytical nature helped him shape his career as a researcher.
Identifying key challenges faced by clients and devising robust, hypothesis-based solutions to empower them with strategic decision-making capabilities come naturally to him. His primary expertise lies in areas such as Market Entry and Expansion Strategy, Feasibility Studies, Competitive Intelligence, and Strategic Transformation.
Holding a degree in Microbiology, Sabyasachi has authored numerous publications and has been cited in journals, including The Journal of mHealth, ITN Online, and Spinal Surgery News.
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-845-579-5705
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube