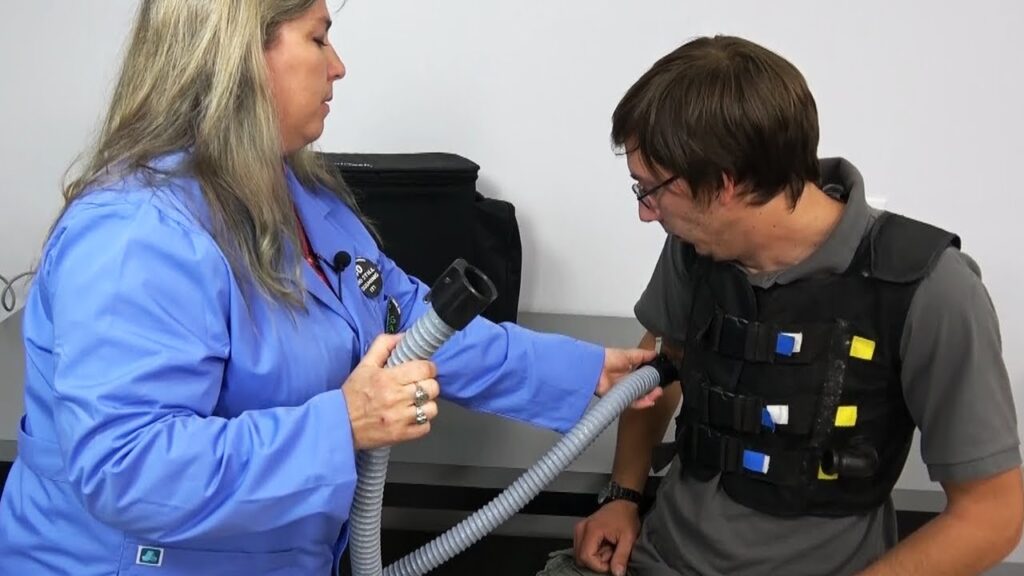
The global airway clearance devices system market is poised for significant growth over the next decade, with an estimated valuation of USD 624.5 million in 2023. According to recent industry forecasts, the market is expected to expand at a compound annual growth rate (CAGR) of 6.2% from 2023 to 2033, reaching an anticipated value of USD 1,137.2 million by 2033.
Airway clearance techniques (ACTs) are essential treatment methods for individuals suffering from disorders such as cystic fibrosis, helping them maintain a healthier lifestyle. These techniques involve physical or mechanical methods to facilitate the removal of tracheobronchial phlegm through the external or internal management of airflow, followed by the expulsion of phlegm via coughing.
The anticipated growth of the airway clearance devices system market is driven by the increasing prevalence of respiratory disorders, particularly cystic fibrosis, and the growing demand for effective treatment solutions. ACTs play a crucial role in improving the quality of life for patients by simplifying the elimination of phlegm and enhancing respiratory function.
Request Your Detailed – Report Sample
In order to maximize outcomes and enhance the patient experience, stakeholders in the respiratory treatment sector are concentrating on innovation, education, and patient-centered care as the global healthcare landscape changes. Airway clearance medicine availability can be increased globally by manufacturers and healthcare providers addressing unmet requirements through investments in clinical evidence collecting, patient support programs, and research and development.
Key Drivers of Market Growth:
- Rising Prevalence of Respiratory Disorders: The increasing number of individuals diagnosed with cystic fibrosis, chronic obstructive pulmonary disease (COPD), and bronchiectasis is driving the demand for airway clearance devices.
- Technological Advancements: Medical device companies are continually innovating and developing more user-friendly and effective airway clearance devices. For instance, Project Fizzyo, a collaboration between Microsoft, Great Ormond Street Hospital, and University College London, gamifies cystic fibrosis treatment, making it more engaging for children.
- Improved Patient Outcomes: Airway clearance devices effectively remove mucus, leading to reduced lung infections and improved lung function for patients.
Competitive Landscape:
To increase their market share significantly, most businesses take part in joint research projects, market expansions, and strategic acquisitions. Allergan plc, Dymedso Inc., Electromed Inc., Ltd., General Physiotherapy Inc., Hill-Rom Holdings, Inc., International Biophysical Corporation, Koninklijke Philips N.V. (Philips), Monaghan Medical Corporation, Inc., PARI GmbH, and Thayer Medical are a few of the well-known companies in the global market for airway clearance devices.
Recent Developments:
- In May 2022, AbbVie, a research-based global biopharmaceutical company, announced that it has completed its acquisition of Allergan plc following receipt of regulatory approval from all government authorities required by the transaction agreement and approval by the Irish High Court.
- In 2021, Baxter International, a renowned MedTech leader, announced that it had completed the acquisition of Hillrom. Baxter paid US$ 156 in cash for each outstanding share of Hillrom common stock for a purchase price of US$ 10.5 million. Including the assumption of Hillrom’s outstanding debt obligations, the enterprise value of the transaction is about US$ 12.5 million
- Thayer Medical has introduced the Quake airway clearance device for people suffering from mucus-producing respiratory conditions. The product delivers comprehensive and convenient secretion clearance therapy that has an easy-to-use, portable, and handheld design.
- Monaghan Medical Corporation has added its oscillating positive expiratory pressure device named Aerobika. This helps in clearing the lungs and serves people with conditions like COPD, cystic fibrosis, and bronchitis
Customization Available: Here
Key Segmentation:
By Product Type:
- Positive Expiratory Pressure
- Intrapulmonary Percussive Ventilation
- Oral High-Frequency Oscillation
- High-Frequency Chest Wall Oscillation
- Flutter
- Incentive Spirometry
By End User:
- Hospitals
- Clinics
- Ambulatory Surgical Centers
Key Regions Covered:
- North America
- The United States
- Canada
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Europe
- Germany
- The United Kingdom
- France
- Spain
- Russia
- Rest of Europe
- Japan
- Asia Pacific Excluding Japan
- China
- India
- Malaysia
- Singapore
- Australia
- Rest of Asia Pacific Excluding Japan (APEJ)
- The Middle East and Africa
- GCC Countries
- Israel
- South Africa
- The Middle East and Africa (MEA)
In-Depth Market Overview: Purchase Now to Access
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-845-579-5705
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube